News
Compliance with the Safety Production Law and Being the First Responsible Person -2022 Jiande Zhengfa Pharmaceutical Co., Ltd. Safety Month Activity Launch Ceremony

A unique 'Goddess Festival'

2021-03-16
Good news: Passed on-site inspection for drug GMP compliance!

The company passed the on-site verification of drug GMP compliance organized by the Zhejiang Provincial Drug Administration in one go
Our company accepts inspection from senior management of Zhendong Group

Zhengfa Pharmaceutical has successfully passed the quality audit of Beijing Zhendong Kangyuan Pharmaceutical Co., Ltd. - promoting cooperation through products and achieving win-win development

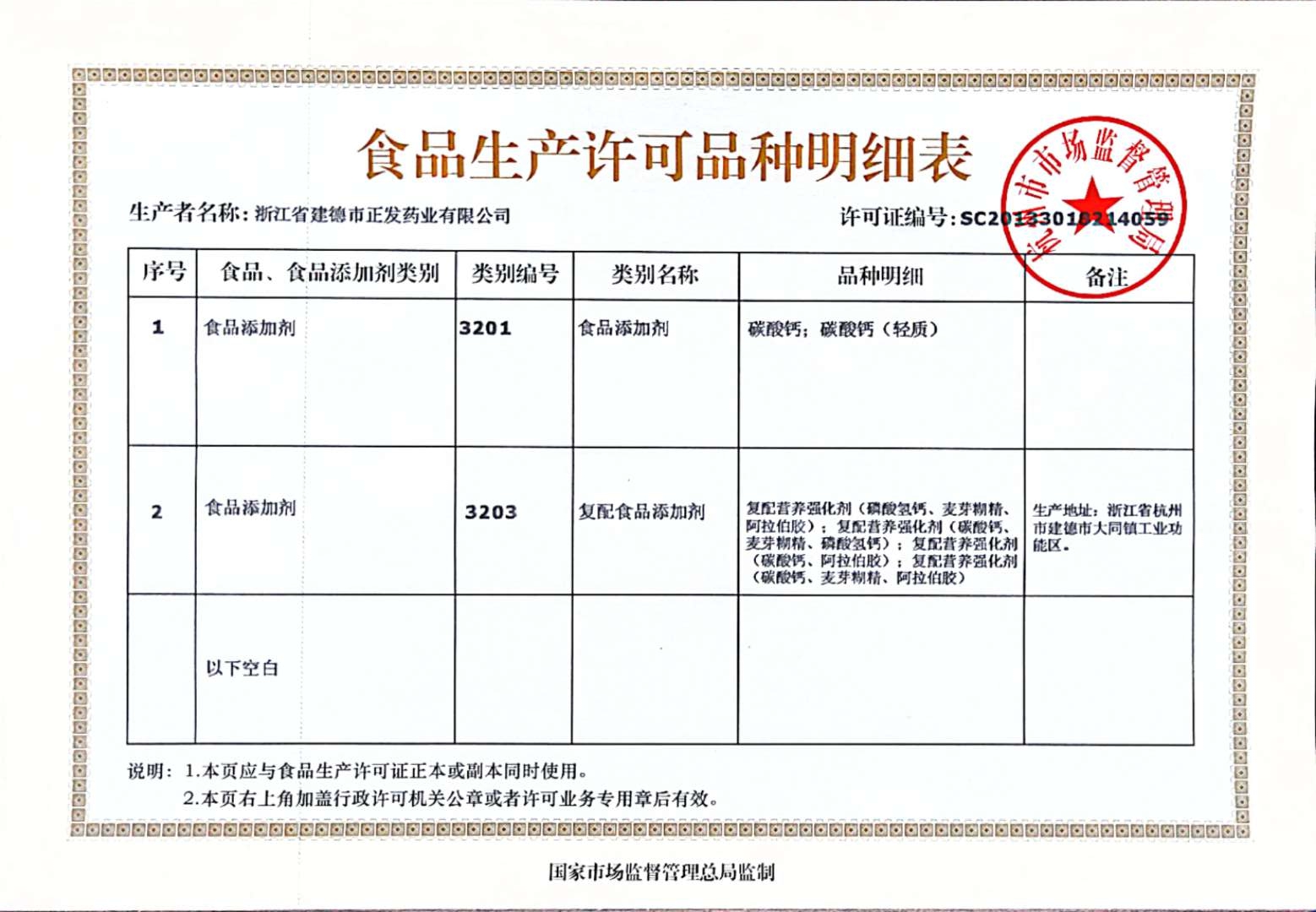
Application of Calcium Carbonate

2020-04-25
Basic knowledge of calcium carbonate
2020-04-25
Actively respond to national policies and study the new version of the Drug Administration Law

2020-04-11
上一页
1
2
下一页

Quick message
Bottom message
Describe:
Contact Information
Address:Industrial Functional Zone, Datong Town, Jiande City, Zhejiang Province
Telephone: 0571-64596398
Phone:15397156319
Email :sales@zhengfa-p.com
Copyright © 2022 Zhejiang Jiande Zhengfa Pharmaceutical Co., Ltd. All rights reserved.{浙ICP备18057609号-1}

